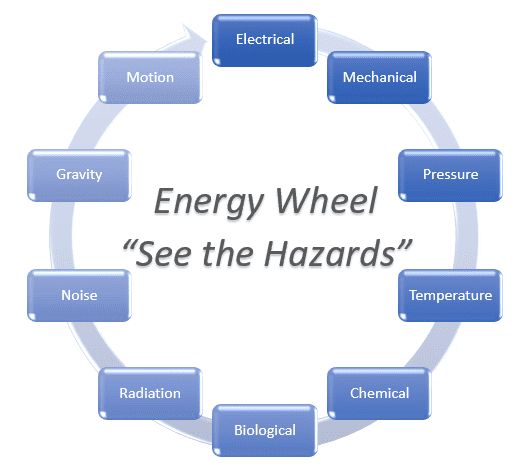
Latent heat is the heat released or absorbed by a chemical substance or a thermodynamic system during a process that occurs without a change in temperature. A typical example is a change of state of matter, meaning a phase transition such as the melting of ice or the boiling of water. In easy words the latent heat of fusion of ice is 80 kcal/kg. The term was introduced around 1750 by Joseph Black. It is derived from the Latin latere (to lie hidden).
Two of the more common forms of latent heat (or enthalpies or energies) encountered are latent heat of fusion (melting) and latent heat of vaporization (boiling). These names describe the direction of energy flow when changing from one phase to the next: from solid to liquid, and to gas.
The term latent heat is derived from the Latin latere, meaning to lie hidden. It was introduced into thermodynamics around 1750 by Joseph Black. James Prescott Joule characterized latent energy as the energy of interaction in a given configuration of particles, i.e. a form of potential energy, and the sensible heat as an energy that was indicated by the thermometer, relating the latter to thermal energy.
A specific latent heat (L) expresses the amount of energy in form of heat (Q) required to completely effect a phase change of a unit of mass (m), usually 1kg, of a substance as an intensive property:
The latent heat of condensation of water in the temperature range from −40 °C to 40 °C is approximated by the following empirical cubic function: