Heat transfer is the exchange of thermal energy between physical systems. The rate of heat transfer is dependent on the temperatures of the systems and the properties of the intervening medium through which the heat is transferred. The three fundamental modes of heat transfer are conduction, convection and radiation. Heat transfer, the flow of energy in the form of heat, is a process by which a system changes its internal energy, hence is of vital use in applications of the First Law of Thermodynamics. Conduction is also known as diffusion, not to be confused with diffusion related to the mixing of constituents of a fluid.
Heat is defined in physics as the transfer of thermal energy across a well-defined boundary around a thermodynamic system. The thermodynamic free energy is the amount of work that a thermodynamic system can perform. Enthalpy is a thermodynamic potential, designated by the letter "H", that is the sum of the internal energy of the system (U) plus the product of pressure (P) and volume (V). Joule is a unit to quantify energy, work, or the amount of heat.
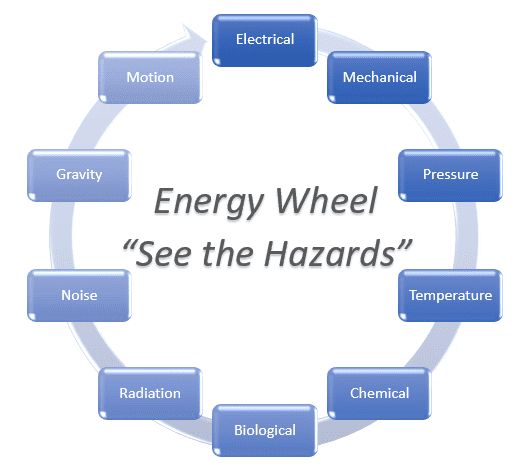
The fundamental modes of heat transfer are:
- Advection
- Advection is the transport mechanism of a fluid from one location to another, and is dependent on motion and momentum of that fluid.
- Conduction or diffusion
- The transfer of energy between objects that are in physical contact. Thermal conductivity is the property of a material to conduct heat and evaluated primarily in terms of Fourier's Law for heat conduction.
- Convection
- The transfer of energy between an object and its environment, due to fluid motion. The average temperature, is a reference for evaluating properties related to convective heat transfer.
- Radiation
- The transfer of energy by the emission of electromagnetic radiation.
By transferring matter, energy—including thermal energy—is moved by the physical transfer of a hot or cold object from one place to another. This can be as simple as placing hot water in a bottle and heating a bed, or the movement of an iceberg in changing ocean currents. A practical example is thermal hydraulics.This can be described by the formula:
On a microscopic scale, heat conduction occurs as hot, rapidly moving or vibrating atoms and molecules interact with neighboring atoms and molecules, transferring some of their energy (heat) to these neighboring particles. In other words, heat is transferred by conduction when adjacent atoms vibrate against one another, or as electrons move from one atom to another. Conduction is the most significant means of heat transfer within a solid or between solid objects in thermal contact. Fluids—especially gases—are less conductive. Thermal contact conductance is the study of heat conduction between solid bodies in contact.
The flow of fluid may be forced by external processes, or sometimes (in gravitational fields) by buoyancy forces caused when thermal energy expands the fluid (for example in a fire plume), thus influencing its own transfer. The latter process is often called "natural convection". All convective processes also move heat partly by diffusion, as well. Another form of convection is forced convection. In this case the fluid is forced to flow by use of a pump, fan or other mechanical means.
Convective cooling is sometimes described as Newton's law of cooling:
In a body of fluid that is heated from underneath its container, conduction and convection can be considered to compete for dominance. If heat conduction is too great, fluid moving down by convection is heated by conduction so fast that its downward movement will be stopped due to its buoyancy, while fluid moving up by convection is cooled by conduction so fast that its driving buoyancy will diminish. On the other hand, if heat conduction is very low, a large temperature gradient may be formed and convection might be very strong.
Thermal radiation occurs through a vacuum or any transparent medium (solid or fluid). It is the transfer of energy by means of photons in electromagnetic waves governed by the same laws. Earth's radiation balance depends on the incoming and the outgoing thermal radiation, Earth's energy budget. Anthropogenic perturbations in the climate system are responsible for a positive radiative forcing which reduces the net longwave radiation loss to space.
Phase transition or phase change, takes place in a thermodynamic system from one phase or state of matter to another one by heat transfer. Phase change examples are the melting of ice or the boiling of water.The Mason equation explains the growth of a water droplet based on the effects of heat transport on evaporation and condensation.
The boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid and the liquid evaporates resulting in an abrupt change in vapor volume.
Condensation occurs when a vapor is cooled and changes its phase to a liquid. During condensation, the latent heat of vaporization must be released. The amount of the heat is the same as that absorbed during vaporization at the same fluid pressure.
Melting is a physical process that results in the phase transition of a substance from a solid to a liquid. The internal energy of a substance is increased, typically by the application of heat or pressure, resulting in a rise of its temperature to the melting point, at which the ordering of ionic or molecular entities in the solid breaks down to a less ordered state and the solid liquefies. An object that has melted completely is molten. Substances in the molten state generally have reduced viscosity with elevated temperature; an exception to this maxim is the element sulfur, whose viscosity increases to a point due to polymerization and then decreases with higher temperatures in its molten state.
Heat transfer can be modeled in the following ways.
Climate models study the radiant heat transfer by using quantitative methods to simulate the interactions of the atmosphere, oceans, land surface, and ice.
The heat equation is an important partial differential equation that describes the distribution of heat (or variation in temperature) in a given region over time. In some cases, exact solutions of the equation are available; in other cases the equation must be solved numerically using computational methods.
Lumped system analysis often reduces the complexity of the equations to one first-order linear differential equation, in which case heating and cooling are described by a simple exponential solution, often referred to as Newton's law of cooling.
Heat transfer has broad application to the functioning of numerous devices and systems. Heat-transfer principles may be used to preserve, increase, or decrease temperature in a wide variety of circumstances. Heat transfer methods are used in numerous disciplines, such as automotive engineering, thermal management of electronic devices and systems, climate control, insulation, materials processing, and power station engineering.
Thermal insulators are materials specifically designed to reduce the flow of heat by limiting conduction, convection, or both. Thermal resistance is a heat property and the measurement by which an object or material resists to heat flow (heat per time unit or thermal resistance) to temperature difference.
A heat exchanger is used for more efficient heat transfer or to dissipate heat. Heat exchangers are widely used in refrigeration, air conditioning, space heating, power generation, and chemical processing. One common example of a heat exchanger is a car's radiator, in which the hot coolant fluid is cooled by the flow of air over the radiator's surface.
Efficient energy use is the goal to reduce the amount of energy required in heating or cooling. In architecture, condensation and air currents can cause cosmetic or structural damage. An energy audit can help to assess the implementation of recommended corrective procedures. For instance, insulation improvements, air sealing of structural leaks or the addition of energy-efficient windows and doors.
- Smart meter is a device that records electric energy consumption in intervals.
- Thermal transmittance is the rate of transfer of heat through a structure divided by the difference in temperature across the structure. It is expressed in watts per square meter per kelvin, or W/m²K. Well-insulated parts of a building have a low thermal transmittance, whereas poorly-insulated parts of a building have a high thermal transmittance.
- Thermostat is a device to monitor and control temperature.
Climate engineering consists of carbon dioxide removal and solar radiation management. Since the amount of carbon dioxide determines the radiative balance of Earth atmosphere, carbon dioxide removal techniques can be applied to reduce the radiative forcing. Solar radiation management is the attempt to absorb less solar radiation to offset the effects of greenhouse gases.
The greenhouse effect is a process by which thermal radiation from a planetary surface is absorbed by atmospheric greenhouse gases, and is re-radiated in all directions. Since part of this re-radiation is back towards the surface and the lower atmosphere, it results in an elevation of the average surface temperature above what it would be in the absence of the gases.
The principles of heat transfer in engineering systems can be applied to the human body in order to determine how the body transfers heat. Heat is produced in the body by the continuous metabolism of nutrients which provides energy for the systems of the body. The human body must maintain a consistent internal temperature in order to maintain healthy bodily functions. Therefore, excess heat must be dissipated from the body to keep it from overheating. When a person engages in elevated levels of physical activity, the body requires additional fuel which increases the metabolic rate and the rate of heat production. The body must then use additional methods to remove the additional heat produced in order to keep the internal temperature at a healthy level.
Evaporative cooling happens when water vapor is added to the surrounding air. The energy needed to evaporate the water is taken from the air in the form of sensible heat and converted into latent heat, while the air remains at a constant enthalpy. Latent heat describes the amount of heat that is needed to evaporate the liquid; this heat comes from the liquid itself and the surrounding gas and surfaces. The greater the difference between the two temperatures, the greater the evaporative cooling effect. When the temperatures are the same, no net evaporation of water in air occurs; thus, there is no cooling effect.
In Quantum Physics laser cooling is used to achieve temperatures of near absolute zero (−273.15 °C, −459.67 °F) of atomic and molecular samples, to observe unique quantum effects that can only occur at this heat level.
- Doppler cooling is the most common method of laser cooling.
- Sympathetic cooling is a process in which particles of one type cool particles of another type. Typically, atomic ions that can be directly laser-cooled are used to cool nearby ions or atoms. This technique allows cooling of ions and atoms that cannot be laser cooled directly.
Magnetic evaporative cooling is a process for lowering the temperature of a group of atoms, after pre-cooled by methods such as laser cooling. Magnetic refrigeration cools below 0.3K, by making use of the magnetocaloric effect.
Radiative cooling is the process by which a body loses heat by radiation. Outgoing energy is an important effect in the Earth's energy budget. In the case of the Earth-atmosphere system, it refers to the process by which long-wave (infrared) radiation is emitted to balance the absorption of short-wave (visible) energy from the Sun. Convective transport of heat and evaporative transport of latent heat both remove heat from the surface and redistribute it in the atmosphere.
Thermal energy storage refers to technologies used to collect and store energy for later use. They can be employed to balance energy demand between day and nighttime. The thermal reservoir may be maintained at a temperature above (hotter) or below (colder) than that of the ambient environment. Applications include later use in space heating, domestic or process hot water, or to generate electricity.