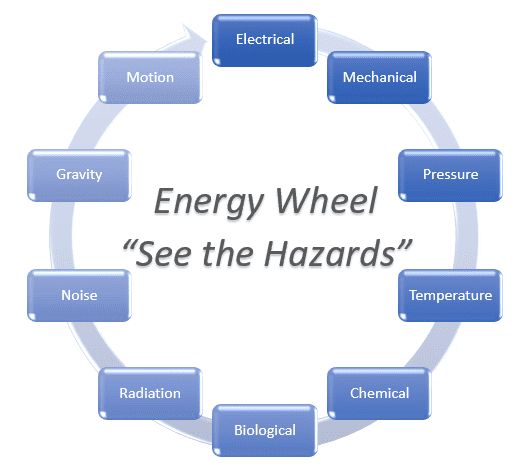
A heat pipe is a heat transfer mechanism that combines the principles of both thermal conductivity and phase transition to efficiently manage the transfer of heat between two solid interfaces.
A typical heat pipe consists of a sealed pipe or tube made of a material with high thermal conductivity such as copper or aluminum at both hot and cold ends. A vacuum pump is used to remove all air from the empty heat pipe, and then the pipe is filled with a fraction of a percent by volume of working fluid (or coolant) chosen to match the operating temperature. Examples of such fluids include water, ethanol, acetone, sodium, or mercury. Due to the partial vacuum that is near or below the vapor pressure of the fluid, some of the fluid will be in the liquid phase and some will be in the gas phase. The use of a vacuum eliminates the need for the working gas to diffuse through any other gas and so the bulk transfer of the vapor to the cold end of the heat pipe is at the speed of the moving molecules. In this sense, the only practical limit to the rate of heat transfer is the speed with which the gas can be condensed to a liquid at the cold end.
Thin planar heat pipes (heat spreaders) have the same primary components as tubular heat pipes. These components are a hermetically sealed hollow vessel, a working fluid, and a closed-loop capillary recirculation system.
Heat pipes employ evaporative cooling to transfer thermal energy from one point to another by the evaporation and condensation of a working fluid or coolant. Heat pipes rely on a temperature difference between the ends of the pipe, and cannot lower temperatures at either end beyond the ambient temperature (hence they tend to equalise the temperature within the pipe).
The general principle of heat pipes using gravity (commonly classified as two phase thermosiphons) dates back to the steam age. The modern concept for a capillary driven heat pipe was first suggested by R.S. Gaugler of General Motors in 1942 who patented the idea. The benefits of employing capillary action were independently developed and first demonstrated by George Grover at Los Alamos National Laboratory in 1963 and subsequently published in the Journal of Applied Physics in 1964.
Publications in 1967 and 1968 by Feldman, Eastman, & Katzoff first discussed applications of heat pipes to areas outside of government concern and that did not fall under the high temperature classification such as; air conditioning, engine cooling, and electronics cooling. These papers also made the first mentions of flexible, arterial, and flat plate heat pipes. 1969 publications introduced the concepts of the rotational heat pipe with its applications to turbine blade cooling and the first discussions of heat pipe applications to cryogenic processes.
Grover and his colleagues were working on cooling systems for nuclear power cells for space craft, where extreme thermal conditions are found. Heat pipes have since been used extensively in spacecraft as a means for managing internal temperature conditions.
Heat pipes are also being widely used in solar thermal water heating applications in combination with evacuated tube solar collector arrays. In these applications, distilled water is commonly used as the heat transfer fluid inside a sealed length of copper tubing that is located within an evacuated glass tube and oriented towards the sun.
Heat pipes are used to dissipate heat on the Trans-Alaska Pipeline System. Without them residual ground heat remaining in the oil as well as that produced by friction and turbulence in the moving oil would conduct down the pipe's support legs. This would likely melt the permafrost on which the supports are anchored. This would cause the pipeline to sink and possibly sustain damage. To prevent this each vertical support member has been mounted with 4 vertical heat pipes.
Heat pipes must be tuned to particular cooling conditions. The choice of pipe material, size and coolant all have an effect on the optimal temperatures in which heat pipes work.
Heat Pipe Science and Technology, Amir Faghri, Taylor and Francis 1995.